How does the valence electron configuration work for sulfur?
Sulfur is an element that can be found on Group 6 and Period 3 on the periodic table with the Atomic Mass 32 and Atomic Number 16. In an electronic configuration, the maximum number of electrons in a given shell can be obtained using the formula 2n², where n represent the number of valence shell. Sulfur/Sulphur (S) It has 6 valence electrons. So, like oxygen it is also very happy with zero formal charge on it. However, unlike oxygen it has more different combinations to get a zero formal charge. One of the combinations is just like oxygen atom (two bonds and two lone pairs) Valence electrons of S = 6. No of bonds = 2. Sulphur has atomic number 16. The electronic configuration of sulphur atom in its ground state is 1s2, 2s2,2p63s2 3p4. Thus it has 2 unpaired electrons,its normal valency is 2, It might engage 4/6 electrons by promoting 1/2 electrons to energetically accessible & vacant 3d orbitals & can exhibit valence 4 &6 also. Sulfur in a sulfate ion has 12 valence electrons: it's original 6 + 6 more from the bonded oxygen. Sulfur's 3s and 3p subshells can only contain 8 of the 12 electrons. The other 4 electrons have entered the sulfur atom's 3d subshell, which is normally empty in elemental sulfur.
1 Answer
Sulfur is located in the 3rd energy level (row) and Group VIA (16th column) of the periodic table. This is the 4th column of the p block,meaning Sulfur must end in a configuration of
The electron configuration for sulfur is
This means that sodium has a valence shell of
The valence level, (highest energy level s and p orbital) is
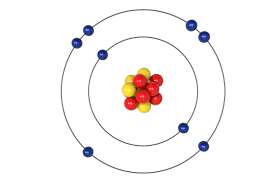
Sulfur has six valence electrons.

I hope this was helpful.
SMARTERTEACHER
Sulphur Atom Valence Electrons

Sulfur Valence Electrons Count
Related questions
